
The TB skin test is a test used to determine if a person is infected with TB bacteria. In this test, a standardized solution made with purified protein derivative (PPD), which is derived from tuberculin, is injected under the skin.
Typically, PPD produces a T-cell mediated delayed-type hypersensitivity reaction if the person has been infected with TB bacteria. In most people who have TB infection, the immune system will recognize the PPD because it is derived from proteins that are excreted by M. tuberculosis growing in specialized media under standardized conditions. PPD is diluted to standard concentration and potency in FDA-approved testing solutions. It takes 2 to 8 weeks after initial infection with TB bacteria for the immune system to be able to react to PPD and for the infection to be detected by the TB skin test.
For Everyone: Testing for Tuberculosis: Skin TestThe TB skin test should be placed (administered) and read by a designated, trained health care provider. Consult with your state and local public health authorities to determine who is authorized to place and read TB skin tests in your state.
Training is essential for health care providers to gain proficiency in the administration and interpretation of the TB skin test.
CDC has free training materials on reading and administering the TB skin test, including a fact sheet, wall chart, and video. Materials are available for download or to order (within the United States).
Keep Reading: Mantoux Tuberculin Skin Test ToolkitCDC has resources to help health care providers talk to patients about TB testing and treatment.
CDC guidelines recommend using TB blood tests to test for TB infection in most cases. However, TB skin tests are an acceptable alternative in situations where a TB blood test is not available, is too costly, or is too burdensome.
Most people can receive a TB skin test. TB skin tests are contraindicated only for people who have had a severe reaction (e.g. necrosis, blistering, anaphylactic shock, or ulcerations to a previous TB skin test). TB skin tests are not contraindicated for any other persons, including infants, children, pregnant people, or people with HIV.
TB skin tests should not be performed on people who have written documentation of a previous positive TB test result (TB blood test or TB skin test) or treatment for TB disease. Most people who have a positive TB test result will continue to have a positive test result. Additional TB skin tests will probably not contribute to medical care, regardless of the result.
Health care providers should take into account the following factors when considering the TB skin test for a patient:
Current CDC guidelines recommend the TB skin test as the method of testing for children younger than 5 years of age, while noting that some experts use TB blood tests in younger children. Health care providers may choose to consult the American Academy of Pediatrics (AAP) guidance on the use of TB blood tests in children.
The BCG vaccine may cause a false-positive TB skin test reaction. There is no reliable way to distinguish a positive TB skin test reaction caused by BCG vaccination from a reaction caused by true TB infection.
When using the TB skin test, people who have been vaccinated with BCG should always be further evaluated for latent TB infection or TB disease as if they were not vaccinated with BCG. TB skin test reactivity caused by BCG vaccine generally wanes with the passage of time, but periodic skin testing may prolong (boost) reactivity in vaccinated people. TB skin test reactions should be interpreted based on risk stratification regardless of BCG vaccination history.
Health care providers treating children who have been vaccinated with BCG should also know that tuberculin reactivity is likely during the first two years after BCG administration to the newborn.
Multiple BCG doses (as practiced in some countries) increases a person's sensitivity to the TB skin test and the duration of positive TB skin test results after BCG administration.
BCG vaccination does not induce positive results when TB blood tests are used. TB blood tests are the preferred test for people who have received the BCG vaccine, including children.
A boosted reaction can occur in previously infected, older adults whose sensitivity to tuberculin has decreased over time. It also can occur in persons of any age who have been vaccinated with BCG or infected with mycobacteria besides M. tuberculosis complex. When given a TB skin test years after infection, these persons may have little or no reaction, giving a negative skin test result. However, the TB skin test may stimulate the immune system, causing a boosted reaction with a positive result after subsequent tests. The TB blood test does not boost subsequent test results.
Two-step testing is a strategy used to reduce the likelihood that a boosted reaction will be misinterpreted as a recent infection if the person has to be tested again. Two-step testing should be used for the initial (baseline) skin testing of persons who will be retested periodically.
If the TB skin test is used for baseline testing of U.S. health care personnel, use two-step testing.
Vaccination with live viruses, including measles, mumps, rubella, oral polio, varicella, and yellow fever may interfere with TB skin test reactions. For persons scheduled to receive a TB skin test, testing should be done:
Vaccination with inactivated viruses doesn't interfere with TB skin test reactions.
COVID-19 vaccination should not be delayed because of testing for TB infection. TB skin tests are not expected to affect the safety or the effectiveness of COVID-19 vaccines. Testing for TB infection with a TB skin test can be done before, during, or after a COVID-19 vaccination visit.
The TB skin test is performed by injecting 0.1 ml of tuberculin purified protein derivative (PPD) into the inner surface of the forearm. The injection should be made with a disposable 27-gauge tuberculin syringe, with the needle bevel facing upward.
The TB skin test is an intradermal injection. When placed correctly, the injection should produce a pale elevation of the skin (a wheal) 6 to 10 mm in diameter.
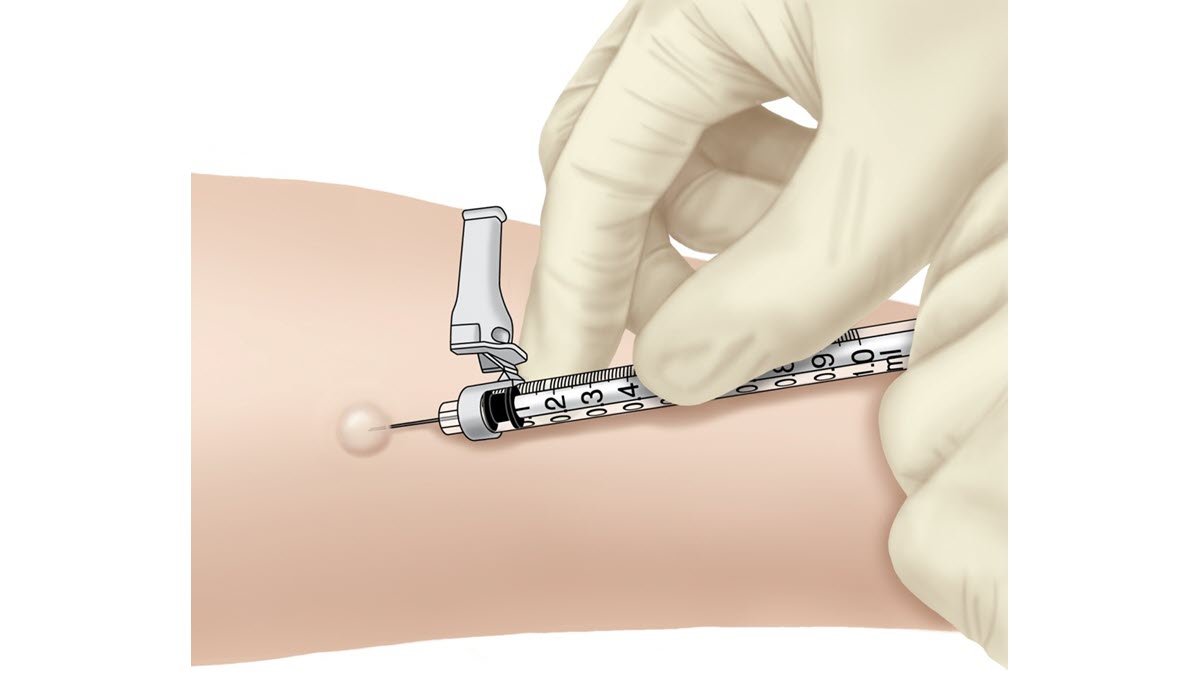
If an incorrect dose (more or less than 0.1 ml) of PPD tuberculin solution is administered during a TB skin test, or if the injection is too deep and does not form a wheal, the person administering the tuberculin should report the incident following the established policies and procedures of the facility or institution in which the incorrect dosage occurred. An incorrect dose or administration of tuberculin invalidates the TB skin test. The test must be repeated, with the new site being at least 2 inches from the previous site.
The skin test reaction should be read between 48 and 72 hours after administration by a health care worker trained to read TB skin results. If a patient has symptoms of TB disease, health care providers should not wait for TB skin test results before starting other diagnostic tests.
A patient who does not return within 72 hours will need to be rescheduled for another skin test. There is no risk associated with repeated TB skin test placements. There is no contraindication to repeating the TB skin test unless a previous TB skin test was associated with a severe reaction. However, repeated skin test placements can cause boosting.
The reaction should be measured in millimeters of the induration (firm swelling). The reader should not measure erythema (redness). The diameter of the indurated area should be measured across the forearm (perpendicular to the long axis).
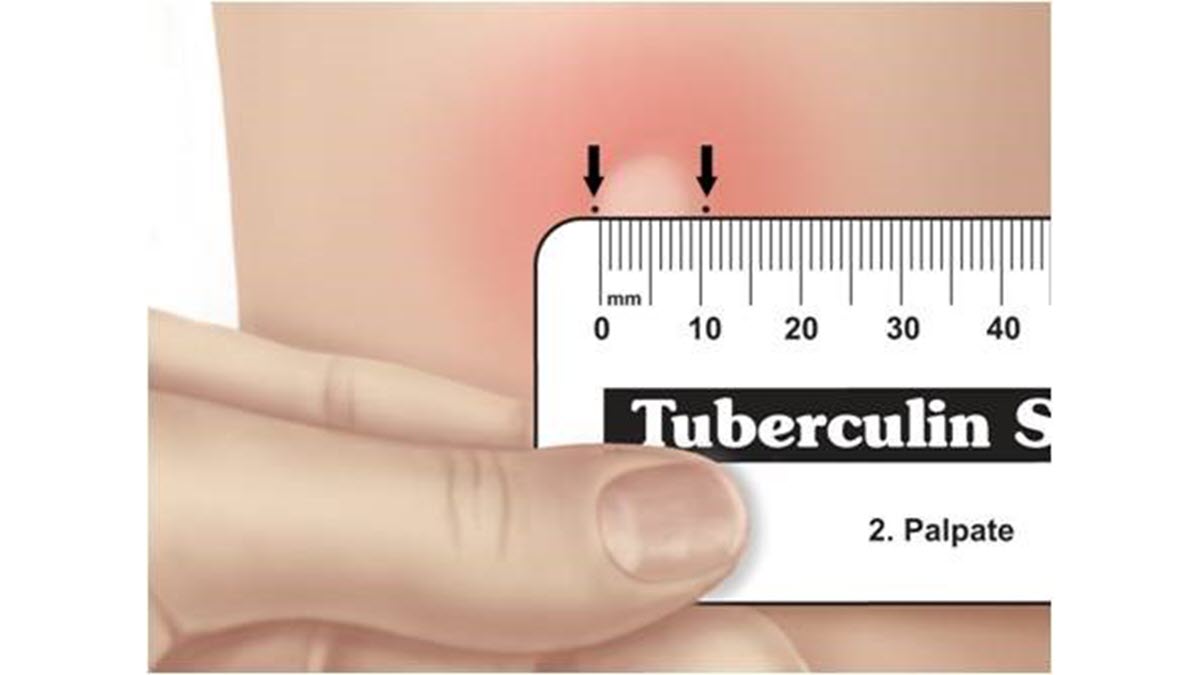
Skin test interpretation depends on two factors:
Some persons may have a positive result from a TB skin test even though they are not infected with TB bacteria. The causes of these false-positive results may include, but are not limited to, the following:
Some persons may have a negative result from a TB skin test even though they are infected with TB bacteria. The reasons for these false-negative results may include, but are not limited to, the following:
Many life-threatening illnesses are associated with false-negative TB skin test results, such as protein-calorie malnutrition, advanced cancer, and TB disease itself, notably miliary TB and TB meningitis. If a false-negative TB skin test result is possible and TB is a concern in one of these situations, consult with a TB expert.
A person with a positive result from a TB skin test or symptoms of TB disease should be evaluated for TB disease. This includes performing:
If latent TB infection is diagnosed, short and convenient treatment regimens are available. Treatment for latent TB infection is 90% effective for preventing the development of TB disease.
It is important to note that a negative TB skin test results does not exclude the diagnosis of TB disease, especially for patients with severe TB illness or infection with HIV.
All persons with clinically active or presumed TB disease should be reported to the local or state health department.
Latent TB infection is reportable in some states and localities. For information on reporting requirements in your jurisdiction, consult your state TB program.