
People enjoy getting together to discuss things, whether it is how your favorite sports team is doing, what the best new movie is, the current politics, or any number of other topics. Often the question is raised about who is right and who is wrong. If the football game is to be played this coming weekend, all we can do is offer opinions as to its outcome. The game has not been played yet, so we don’t know who will actually win.

The ancient Greek philosophers did a lot of discussing, with part of their conversations concerning the physical world and its composition. There were different opinions about what made up matter. Some felt one thing was true while others believed another set of ideas. Since these scholars did not have laboratories and had not developed the idea of the experiment, they were left to debate. Whoever could offer the best argument was considered right. However, often the best argument had little to do with reality.

Figure 1. Into how small of pieces can you divide a grain of sand?
One of the on-going debates had to do with sand. The question posed was: into how small of pieces can you divide a grain of sand? The prevailing thought at the time, pushed by Aristotle, was that the grain of sand could be divided indefinitely, that you could always get a smaller particle by dividing a larger one and there was no limit to how small the resulting particle could be.
Since Aristotle was such an influential philosopher, very few people disagreed with him. However, there were some philosophers who believed that there was a limit to how small a grain of sand could be divided. One of these philosophers was Democritus (~460-~370 B.C.), often referred to as the “laughing philosopher” because of his emphasis on cheerfulness. He taught that there were substances called atoms and that these atoms made up all material things. The atoms were unchangeable, indestructible, and always existed.
Figure 2. Democritus.
The word “ atom ” comes from the Greek atomos and means “indivisible.” The atomists of the time (Democritus being one of the leading atomists) believed there were two realities that made up the physical world: atoms and void. There was an infinite number of atoms, but different types of atoms had different sizes and shapes. The void was the empty space in which the atoms moved and collided with one another. When these atoms collided with one another, they might repel each other or they might connect in clusters, held together by tiny hooks and barbs on the surface of the atoms.
Aristotle disagreed with Democritus and offered his own idea of the composition of matter. According to Aristotle, everything was composed of four elements: earth, air, fire, and water. The theory of Democritus explained things better, but Aristotle was more influential, so his ideas prevailed. We had to wait almost two thousand years before scientists came around to seeing the atom as Democritus did.
It is very interesting that Democritus had the basic idea of atoms, even though he had no experimental evidence to support his thinking. We now know more about how atoms hold together in “clusters” (compounds), but the basic concept existed over two thousand years ago. We also know that atoms can be further subdivided, but there is still a lower limit to how small we can break up that grain of sand.
Use the link below to answer the following questions:
philosopher: People who do a lot of discussing and debate, with part of their conversations concerning the physical world and its composition.
atom: The philosopher Democritus (~460-~370 B.C.), taught that there were substances called atoms and that these atoms made up all material things. The atoms were unchangeable, indestructible, and always existed.
State the law of conservation of mass.

The following situation happens all too often. You have taken apart a piece of equipment to clean it up. When you put the equipment back together, somehow you have an extra screw or two. Or you find out that a screw is missing that was a part of the original equipment. In either case, you know something is wrong. You expect to end up with the same amount of material that you started with, not with more or less than what you had originally.
By the late 1700s, chemists accepted the definition of an element as a substance that cannot be broken down into a simpler substance by ordinary chemical means. It was also clear that elements combine with one another to form more complex substances called compounds. The chemical and physical properties of these compounds are different than the properties of the elements from which they were formed. There were questions about the details of these processes.
In the 1790s, a greater emphasis began to be placed on the quantitative analysis of chemical reactions. Accurate and reproducible measurements of the masses of reacting elements and the compounds they form led to the formulation of several basic laws . One of these is called the law of conservation of mass , which states that during a chemical reaction, the total mass of the products must be equal to the total mass of the reactants . In other words, mass cannot be created or destroyed during a chemical reaction, but is always conserved.
As an example, consider the reaction between silver nitrate and sodium chloride. These two compounds will dissolve in water to form silver chloride and sodium nitrate. The silver chloride does not dissolve in water, so it forms a solid that we can filter off. When we evaporate the water, we can recover the sodium nitrate formed. If we react 58.5 grams of sodium chloride with 169.9 grams of silver nitrate, we start with 228.4 grams of materials. After the reaction is complete and the materials separated, we find that we have formed 143.4 grams of silver chloride and 85.0 grams of sodium nitrate, giving us a total mass of 228.4 grams for the products. So, the total mass of reactants equals the total mass of products, a proof of the law of conservation of mass.
Watch this video for a demonstration on the Law of Conservation of Mass
Use the link below to answer the following questions:
State the law of definite proportions.

We use electricity for many purposes, from cooking to powering our televisions to charging our cell phones. Wherever we travel in the United States, we want electricity to be available. What we also want (although we usually don’t think about it) is for the electricity supply to be the same wherever we go. We want the same voltage (110 volts for the U.S.) to come from the outlet to whatever we plug in. If the voltage is less, the system will not work. If it is more, the equipment will be damaged. We want a definite amount of voltage – no more and no less.
The discovery that mass was always conserved in chemical reactions was soon followed by the law of definite proportions , which states that a given chemical compound always contains the same elements in the exact same proportions by mass. As an example, any sample of pure water contains 11.19% hydrogen and 88.81% oxygen by mass. It does not matter where the sample of water came from or how it was prepared. Its composition, like that of every other compound, is fixed.
Another example is carbon dioxide. This gas is produced from a variety of reactions, often by the burning of materials. The structure of the gas consists of one atom of carbon and two atoms of oxygen. Carbon dioxide production is of interest in many areas, from the amount we breather out to the amount of the gas produced by burning wood or fossil fuels. By knowing the exact composition of carbon dioxide, we can make predictions as to the effects of different chemical processes.

Figure 4. Carbon dioxide is produced during the burning process.
Watch the video and answer the questions:
law of definite proportions: States that a given chemical compound always contains the same elements in the exact same proportions by mass.
State the law of multiple proportions.

Just from the words themselves, the astute Latin-speaking scholar can tell that, whatever it is made of, the unicycle has one of them ( uni = “one”) and the bicycle has two ( bi = “two”). From the picture to the right, we get additional information that helps us tell the two apart.
The unicycle has one wheel and the bicycle has two. In particular, they are made up of the same materials and the only significant difference is the number of wheels on the two vehicles.
Now: how many wheels on a tricycle?
Once the idea that elements combined in definite proportions to form compounds was established, experiments also began to demonstrate that the same pairs of certain elements could combine to form more than one compound. Consider the elements carbon and oxygen. Combined in one way, they form the familiar compound called carbon dioxide. In every sample of carbon dioxide, there is 32.0 g of oxygen present for every 12.0 g of carbon. By dividing 32.0 by 12.0, this simplifies to a mass ratio of oxygen to carbon of 2.66 to 1. There is another compound that forms from the combination of carbon and oxygen called carbon monoxide. Every sample of carbon monoxide contains 16.0 g of oxygen for every 12.0 g of carbon. This is a mass ratio of oxygen to carbon of 1.33 to 1. In the carbon dioxide, there is exactly twice as much oxygen present as there is in the carbon monoxide. This example illustrates the law of multiple proportions : Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.

Figure 5. Carbon can form two different compounds with oxygen.
In carbon monoxide, on the left, there is 1.333 g of oxygen for every 1 g of carbon. In carbon dioxide, on the right, there is 2.666 g of oxygen for every gram of carbon. So the ratio of oxygen in the two compounds is 1:2, a small whole number ratio.
The difference between carbon monoxide and carbon dioxide is significant. Carbon monoxide is a deadly gas, formed from the incomplete combustion of some carbon-containing materials (such as wood and gasoline). This compound will attach to hemoglobin in the red blood cell and block the binding of oxygen to those cells. If oxygen does not bind, it cannot be carried to the cells of the body where it is needed and death can occur. Carbon dioxide, on the other hand, is not toxic like carbon monoxide is. However, it can displace oxygen in systems since it is heavier. Carbon dioxide fire extinguishers cut off the flow of oxygen in a fire, putting out the fire.
Watch the video at the link below or read the transcript and answer the following questions:
law of multiple proportions: Whenever the same two elements form more than one compound, the different masses of one element that combine with the same mass of the other element are in the ratio of small whole numbers.

One of the fundamental laws of chemistry deals with the fact that we cannot (using chemical means) create or destroy matter. When a reaction is run, the number of atoms of each specific type must be the same on both sides of the equation. For some materials, it turns out that one element can combine with a second element in more than one ratio. Carrying out mass ratio calculations helped establish the law of multiple proportions.
Copper reacts with chlorine to form two compounds. Compound A consists of 4.08 g of copper for every 2.28 g of chlorine. Compound B consists of 7.53 g of copper for every 8.40 g of chlorine. What is the lowest whole number mass ratio of copper that combines with a given mass of chlorine?
Apply the law of multiple proportions to the two compounds. For each compound, find the grams of copper that combine with 1.00 g of chlorine by dividing the mass of copper by the mass of chlorine. Then find the ratio of the masses of copper in the two compounds by dividing the larger value by the smaller value.
Compare the masses of copper per gram of chlorine in the two samples.
![]()
The mass ratio of copper per gram of chlorine in the two compounds is 2:1.
The ratio is a small whole-number ratio. For a given mass of chlorine, compound A contains twice the mass of copper as does compound B.

Use the link below to answer the following questions:
List the components of Dalton’s atomic theory.

Pick a little, talk a little, pick a little, talk a little,
Cheep cheep cheep, talk a lot, pick a little more
These lyrics from the musical “Music Man” summed up the way science was done for centuries. OK, the lyrics referred to a group of gossiping ladies, but the outcome was the same.
The Greek and Roman philosophers debated, discussed, and sometimes even attacked one another. But the mode of discovery was talk. There was no experimentation – the idea had not been thought of yet. So science did not develop very far and there was no reliable way to establish what was true and what was false.

Figure 7. Dalton.
While it must be assumed that many more scientists, philosophers and others studied the composition of matter after Democritus, a major leap forward in our understanding of the composition of matter took place in the 1800s with the work of the British scientist John Dalton. He started teaching school at age twelve, and was primarily known as a teacher. In his twenties, he moved to the growing city of Manchester, where he was able to pursue some scientific studies. His work in several areas of science brought him a number of honors. When he died, over 40,000 people in Manchester marched at his funeral.
Dalton studied the weights of various elements and compounds. He noticed that matter always combined in fixed ratios based on weight, or volume in the case of gases. Chemical compounds always contain the same proportion of elements by mass, regardless of amount, which provided further support for Proust’s law of definite proportions. Dalton also observed that there could be more than one combination of two elements.
From his experiments and observations, as well as the work from peers of his time, Dalton proposed a new theory of the atom. This later became known as Dalton’s atomic theory. The general tenets of this theory were as follows:
Dalton’s atomic theory has been largely accepted by the scientific community, with the exception of three changes. We know now that (1) an atom can be further sub-divided, (2) all atoms of an element are not identical in mass, and (3) using nuclear fission and fusion techniques, we can create or destroy atoms by changing them into other atoms.
Figure 8. Dalton’s symbols.
Use the link below to do the exercise. Read the sections and take the quiz at the end.

The TV set seen above is becoming harder and harder to find these days. The main reason is because they are older and based on outdated technology. The new TV sets are flat screen technology that take up less space and give better picture quality, especially with the advent of high-definition broadcasting.
The technology used in the older TV sets used cathode ray tubes. A beam of electrons was sprayed to a picture tube which was treated to react with the electrons to produce an image. Similar CRT devices were used in computer monitors, now also replaced by flat screen monitors.
The first discovery of a subatomic particle was a result of experiments into the nature of the relationship between electricity and matter.
The first cathode ray tube prototype was developed by Heinrich Geissler, a German glassblower and physicist. He used a mercury pump to create a vacuum in a tube. Geissler explored a number of techniques to remove air from the tube and to prevent leaks, as well as ways to get good connections of the wires in the tubes.
In 1878, Sir William Crookes, a British scientist, displayed the first cathode rays using a modification of the Geissler apparatus. His major contribution to construction of the tube was to develop ways to evacuate almost all the air from the tube. Crookes also carried out many experiments using more reliable equipment to confirm earlier finding about the properties of cathode rays. He discovered two things which supported the hypothesis that the cathode ray consisted of a stream of particles:


Figure 9. The cathode ray tube was first invented by Sir William Crookes.
Crookes’ work opened the door to a number of important discoveries. Other scientists were able to demonstrate that the “cathode ray” was actually a stream of electrons . In 1897, Karl Ferdinand Braun developed the first oscilloscope, using a cathode ray tube to see an electrical pulse as it passed through the instrument. The invention of television would not have been possible without the cathode ray tube. Work with a modified system led to the discovery of X-rays in 1895 by the German physicist Wilhelm Roentgen. This simple device has led to major advances in science and technology.
Use the link below to answer the following questions:
Describe evidence from cathode ray tube experiments that demonstrate properties of the electron.

In a power outage all your electrical equipment suddenly stops working. The radio was on just a minute ago and now it is silent. What happened? Somewhere between a power generator and your electrical device was an interruption. Power stopped flowing through the wires and into your radio. That “power” turns out to be electrons that move through the wires and cause an electrical current to flow.
As the nineteenth century began to draw to a close, the concept of atoms was well-established. We could determine the mass of different atoms and had some good ideas about the atomic composition of many compounds. Dalton’s atomic theory held that atoms were indivisible, so scientists did not ask questions about what was inside the atom – it was solid and could not be broken down further. But then things began to change.
In 1897, English physicist J.J. Thomson (1856-1940) experimented with a device called a cathode ray tube, in which an electric current was passed through gases at low pressure. A cathode ray tube consists of a sealed glass tube fitted at both ends with metal disks called electrodes. The electrodes are then connected to a source of electricity. One electrode, called the anode , becomes positively charged while the other electrode, called the cathode , becomes negatively charged. A glowing beam (the cathode ray) travels from the cathode to the anode.

Earlier investigations by Sir William Crookes and others had been carried out to determine the nature of the cathode ray. Thomson modified and extended these experiments in an effort to learn about these mysterious rays. He discovered two things, which supported the hypothesis that the cathode ray consisted of a stream of particles.
In order to determine if the cathode ray consisted of charged particles, Thomson used magnets and charged plates to deflect the cathode ray. He observed that cathode rays were deflected by a magnetic field in the same manner as a wire carrying an electric current, which was known to be negatively charged. In addition, the cathode ray was deflected away from a negatively charged metal plate and towards a positively charged plate.

Thomson knew that opposite charges attract one another, while like charges repel one another. Together, the results of the cathode ray tube experiments showed that cathode rays are actually streams of tiny negatively charged particles moving at very high speeds. While Thomson originally called these particles corpuscles, they were later named electrons.
![]() of the electron. In units of coulombs to grams, this value is 1.8 × 10 8 Coulombs/gram. He found that this value was a constant and did not depend on the gas used in the cathode ray tube or on the metal used as the electrodes. He concluded that electrons were negatively charged subatomic particles present in atoms of all elements.
of the electron. In units of coulombs to grams, this value is 1.8 × 10 8 Coulombs/gram. He found that this value was a constant and did not depend on the gas used in the cathode ray tube or on the metal used as the electrodes. He concluded that electrons were negatively charged subatomic particles present in atoms of all elements.
Watch a video of a cathode ray tube experiment:
Use the link below to answer the following questions:
Describe the oil drop experiment.
How tall are you? How much do you weigh? Questions like these are easy to answer because we have the tools to make the measurements. A yard stick or tape measure will suffice to measure height. You can stand on a bathroom scale and determine your weight.
But it is a very different matter to measure properties of objects that we cannot see with the naked eye. If we want to measure the size of a germ, we have to use a microscope. To learn the size of a single molecule, we have to use even more sophisticated instruments. So how would we measure something even smaller than a molecule, even smaller than an atom?

Figure 10. Robert Millikan.
The man who measured properties of the electron was Robert Millikan (1868–1953). He taught himself physics while a student at Oberlin College since there was nobody on the faculty to instruct him in this field. Millikan completed postgraduate research training in the U.S. and in Germany. His studies on the properties of the electron proved to be of great value in many areas of physics and chemistry.
Millikan carried out a series of experiments between 1908 and 1917 that allowed him to determine the charge of a single electron, famously know as the oil drop experiment.
He sprayed tiny drops of oil into a chamber. In his first experiment, he simply measured how fast the drops fell under the force of gravity. He could then calculate the mass of the individual drops. Then he sprayed oil drops and applied an electrical charge to them by shining X-rays up through the bottom of the apparatus. The X-rays ionized the air, causing electrons to attach to the oil drops. The oil drops picked up static charge and were suspended between two charged plates. Millikan was able to observe the motion of the oil drops with a microscope and found that the drops lined up in a specific way between the plates, based on the number of electric charges they had acquired.

Figure 11. Oil drop experiment.
Millikan used the information to calculate the charge of an electron. He determined the charge to be 1.5924 × 10 –19 C, where C stands for coulomb , which is one ampere/second. Today the accepted value of the charge of an electron is 1.602176487 × 10 –19 C. Millikan’s experimental value proved very accurate; it is within 1% of the currently accepted value. Millikan later used the information from his oil drop experiment to calculate the mass of an electron. The accepted value today is 9.10938215 ×10 –31 kg. The incredibly small mass of the electron was found to be approximately 1/1840 the mass of a hydrogen atom. Therefore, scientists realized that atoms must contain another particle that carries a positive charge and is far more massive than the electron.
Use the link below to answer the following questions:
Describe Goldstein’s research that led to the discovery of the proton.

Describing what we can see is a fairly easy matter. If we are asked to describe the sports car illustrated below, we could all quickly come up with a fairly accurate description. A person knowledgeable about cars would include more details, but everyone would have the basic information in their description.
What makes the description easy to come up with? We can see it, we have a common language to describe it (size, color, construction), and we have a basic idea of what it is (a car, not a house or a tree). Scientists have far more difficulty in describing things they cannot see. There is no way to look directly at an atom and see its detailed structure. When a discovery is first made, there is often no language to use to tell others exactly what it is. This was the problem in talking about the atom and its structure.
Research builds upon itself – one piece connects to another. Sometimes the puzzle doesn’t seem to make sense because some of the pieces are missing at the moment. Each finding gives a clearer picture of the whole and also raises new questions. The detective work that led to the discovery of the proton was built upon finding pieces to the puzzle and putting them together in the right way.
The electron was discovered using a cathode ray tube. An electric current was passed from the cathode (the negative pole) to the anode (positive pole). Several experiments showed that particles were emitted at the cathode and that these particles had a negative charge. These experiments demonstrated the presence of electrons.

Figure 12. JJ Thomson’s experiment with cathode rays.
If cathode rays are electrons that are given off by the metal atoms of the cathode, then what remains of the atoms that have lost those electrons? We know several basic things about electrical charges. They are carried by particles of matter. Millikan’s experiment showed that they exist as whole-number multiples of a single basic unit. Atoms have no overall electrical charge, meaning that each and every atom contains an exactly equal number of positively and negatively charged particles. A hydrogen atom is the simplest kind of atom with only one electron. When that electron is removed, a positively charged particle should remain.
In 1886 Eugene Goldstein (1850–1930) discovered evidence for the existence of this positively charged particle. Using a cathode ray tube with holes in the cathode, he noticed that there were rays traveling in the opposite direction from the cathode rays. He called these canal rays and showed that they were composed of positively charged particles. The proton is the positively charged subatomic particle present in all atoms. The mass of the proton is about 1840 times the mass of the electron.
Use the link below to answer the following questions:

The most famous detective in history and literature never existed. Sherlock Holmes was the creation of the British author Sir Arthur Conan Doyle. This mythical person had capabilities far beyond those of mere mortals. Holmes was capable of spotting the tiniest clue, the smallest piece of evidence to solve the crime. He could link all sorts of seemingly irrelevant data into a coherent whole to clear up whatever mystery he was dealing with.
Clues are generally considered to involve the presence of something—a footprint, a piece of fabric, a bloodstain, something tangible that we can measure directly. The discoveries of the electron and the proton were accomplished with the help of those kinds of clues. Cathode ray tube experiments showed both the negatively charged electrons emitted by the cathode and the positively charged proton (also emitted by the cathode). The neutron was initially found not by a direct observation, but by noting what was not found.
Research had shown the properties of the electron and the proton. Scientists learned that approximately 1837 electrons weighed the same as one proton. There was evidence to suggest that electrons went around the heavy nucleus composed of protons. Charge was balanced with equal numbers of electrons and protons which made up an electrically neutral atom. But there was a problem with this model—the atomic number (number of protons) did not match the atomic weight. In fact, the atomic number was usually about half the atomic weight. This indicated that something else must be present. That something must weigh about the same as a proton, but could not have a charge – this new particle had to be electrically neutral.
In 1920 Ernest Rutherford tried to explain this phenomenon. He proposed that the “extra” particles were combinations of protons and electrons in the nucleus. These new particles would have a mass very similar to a proton, but would be electrically neutral since the positive charge of the proton and the negative charge of the electron would cancel each other out.
In 1930 German researchers bombarded the element beryllium with alpha particles (helium nuclei containing two protons and two neutrons with a charge of +2). The particles produced in this process had strong penetrating power, which suggested they were fairly large. In addition, they were not affected by a magnetic field, so they were electrically neutral. The French husband-wife research team of Frederic and Irene Joliot-Curie used these new “rays” to bombard paraffin, which was rich in protons. The unknown particles produced a large emission of protons from the paraffin.
The English physicist James Chadwick (1891–1974) repeated these experiments and studied the energy of these particles. By measuring velocities, he was able to show that the new particle has essentially the same mass as a proton. So we now have a third subatomic particle with a mass equal to that of a proton, but with no charge. This particle is called the neutron. Chadwick won the Nobel Prize in Physics in 1935 for his research.
Neutrons can be used in a variety of ways. One important use is in nuclear fission to produce new isotopes. A neutron will collide with a large atom (such as uranium) and cause it to split into smaller atoms, such as in the Figure 13.

Figure 13. A neutron collides with a large atom, splitting it into smaller atoms.
Nuclear reactors utilize chain reactions involving neutrons to heat water which drive turbines for the generation of electricity. When a neutron collides with a large atom, the atom splits with the release of more neutrons and also a large amount of energy. The energy converts water to steam for the turbine, while the neutrons serve to continue the chain reaction (see Figure 14).

Figure 14. How nuclear fission produces new isotopes.
Read this page on the neutron to answer the following questions:

Millions of children over the years have enjoyed building models—this model airplane is one example of the types of models that can be constructed. Perhaps sixty years ago the models were made of balsa wood, a very light material. Parts would be cut by hand, carefully glued together, and then covered with paper or other fabric.
The development of plastics made the construction of model aircraft much simpler in many respects. And, the end-product is more durable and damage-proof.
A model serves a useful purpose—it gives us an idea of what the real thing is like. The model plane seen above has wings, a tail, and an engine just like the real thing. This model also has a propeller, as is the case with most small planes and some smaller passenger planes. However, the model is not the real thing. We certainly cannot fly people or cargo in the model (besides maybe a tiny mouse), but we can get some idea of what a real plane looks like and how it works.
Science uses many models to explain ideas. We model the electron as a very small particle with a negative charge. That gives us a picture, but a very incomplete one. This picture works fine for most chemists, but is inadequate for a physicist. Models give us a start toward understanding structures and processes, but certainly are not a complete representation of the entity we are examining.
The electron was discovered by J.J. Thomson in 1897. The existence of protons was also known, as was the fact that atoms were neutral in charge. Since the intact atom had no net charge and the electron and proton had opposite charges, the next step after the discovery of subatomic particles was to figure out how these particles were arranged in the atom. This is a difficult task because of the incredibly small size of the atom. Therefore, scientists set out to design a model of what they believed the atom could look like. The goal of each atomic model was to accurately represent all of the experimental evidence about atoms in the simplest way possible.
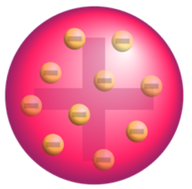
Figure 15. The “plum pudding” model.
Following the discovery of the electron, J.J. Thomson developed what became known as the “ plum pudding ” model in 1904. Plum pudding is an English dessert similar to a blueberry muffin. In Thomson’s plum pudding model of the atom, the electrons were embedded in a uniform sphere of positive charge like blueberries stuck into a muffin. The positive matter was thought to be jelly- like or a thick soup. The electrons were somewhat mobile. As they got closer to the outer portion of the atom, the positive charge in the region was greater than the neighboring negative charges and the electron would be pulled back more toward the center region of the atom.
However, this model of the atom soon gave way to a new model developed by New Zealander Ernest Rutherford (1871-1937) about five years later. Thomson did still receive many honors during his lifetime, including being awarded the Nobel Prize in Physics in 1906 and a knighthood in 1908.
Use the link below to answer the following questions:

As we look at the world around us, it looks pretty solid. We hit a wall with our hand and the hand stops – it does not (normally) go through the wall. We think of matter as occupying space. But there is a lot of empty space in matter. In fact, most of the matter is empty space.
In 1911, Rutherford and coworkers Hans Geiger and Ernest Marsden initiated a series of groundbreaking experiments that would completely change the accepted model of the atom. They bombarded very thin sheets of gold foil with fast moving alpha particles . Alpha particles, a type of natural radioactive particle, are positively charged particles with a mass about four times that of a hydrogen atom.

Figure 16. (A) The experimental setup for Rutherford’s gold foil experiment: A radioactive element that emitted alpha particles was directed toward a thin sheet of gold foil that was surrounded by a screen which would allow detection of the deflected particles. (B) According to the plum pudding model (top) all of the alpha particles should have passed through the gold foil with little or no deflection. Rutherford found that a small percentage of alpha particles were deflected at large angles, which could be explained by an atom with a very small, dense, positively-charged nucleus at its center (bottom).
According to the accepted atomic model, in which an atom’s mass and charge are uniformly distributed throughout the atom, the scientists expected that all of the alpha particles would pass through the gold foil with only a slight deflection or none at all. Surprisingly, while most of the alpha particles were indeed undeflected, a very small percentage (about 1 in 8000 particles) bounced off the gold foil at very large angles. Some were even redirected back toward the source. No prior knowledge had prepared them for this discovery. In a famous quote, Rutherford exclaimed that it was “as if you had fired a 15-inch [artillery] shell at a piece of tissue paper and it came back and hit you.”
Rutherford needed to come up with an entirely new model of the atom in order to explain his results. Because the vast majority of the alpha particles had passed through the gold, he reasoned that most of the atom was empty space. In contrast, the particles that were highly deflected must have experienced a tremendously powerful force within the atom. He concluded that all of the positive charge and the majority of the mass of the atom must be concentrated in a very small space in the atom’s interior, which he called the nucleus. The nucleus is the tiny, dense, central core of the atom and is composed of protons and neutrons.
Rutherford’s atomic model became known as the nuclear model . In the nuclear atom, the protons and neutrons, which comprise nearly all of the mass of the atom, are located in the nucleus at the center of the atom. The electrons are distributed around the nucleus and occupy most of the volume of the atom. It is worth emphasizing just how small the nucleus is compared to the rest of the atom. If we could blow up an atom to be the size of a large professional football stadium, the nucleus would be about the size of a marble.
Rutherford’s model proved to be an important step towards a full understanding of the atom. However, it did not completely address the nature of the electrons and the way in which they occupied the vast space around the nucleus. It was not until some years later that a full understanding of the electron was achieved. This proved to be the key to understanding the chemical properties of elements.
Watch a video that explains the gold foil experiment:
Use the link below to answer the following questions: